A new step towards hydrogen production at sea. But does it really work?
Some days the winds are so turbulent or the sun shines so profusely that managers of wind farms or solar plants don’t know where to go with their sustainable electricity. Caching is an option and this can be done with hydrogen, for example. You can make this gas by splitting water molecules into hydrogen and oxygen using excess electricity.
This so-called electrolysis appears to be the perfect solution for offshore wind farms. After all, there is a lot of water out there. But it doesn’t work like that. Sea water contains all kinds of chemicals. Salt, of course, but also other minerals that can seriously disrupt the electrolysis process. This is why seawater must be purified first, a particularly energy-intensive process that greatly reduces hydrogen storage efficiency.
the anode and the cathode
Researchers at Stanford University in California presented a technique this week that allows them to part seawater directly. This division occurs with a positive and negative electrode, called the anode and the cathode. Since water molecules sometimes split briefly into a positively charged hydrogen ion and a negatively charged residual ion (hydroxide), hydrogen ions will flow to the negative electrode and hydroxide ions to the positive electrode. There it is converted into hydrogen and oxygen gas respectively.
But other positively or negatively charged ions will also swim to those electrodes, where they will turn into harmful substances or will corrode the electrodes. To prevent this, the Americans installed two membranes in their system. These membranes hold all ions except hydrogen and hydroxide.
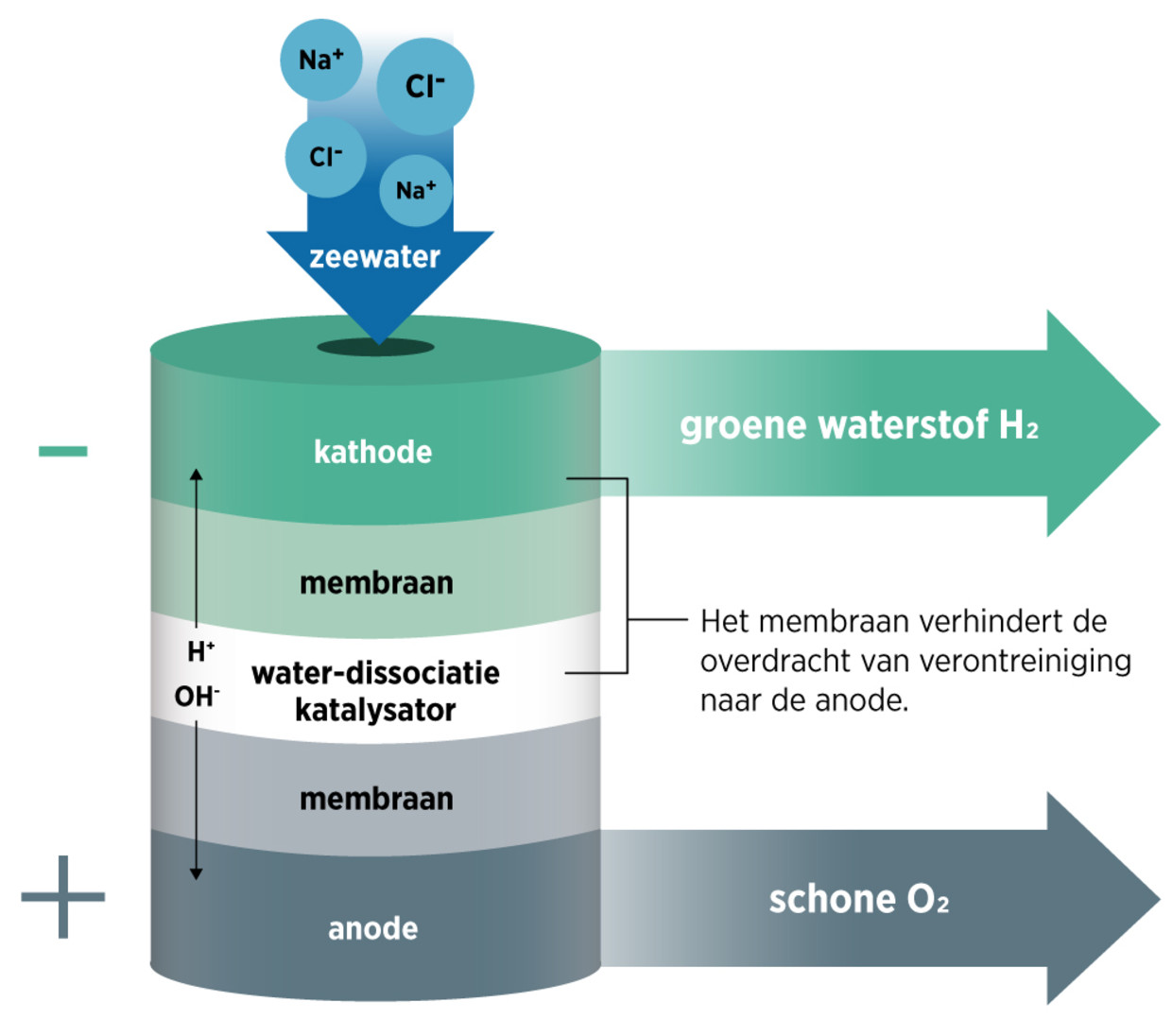
That is, this is the intention. The researchers focused on chloride, the negative ion that forms salt with sodium. You don’t want chloride near the anode. Then chlorine or bleach gas is generated. This is why there are other negative ions on the membrane that prevent chlorine from passing through. This is almost entirely successful, they wrote this week in the Commerce Gazette Joule.
The success gave them confidence that the separation would also work with other harmful ions. They believe that efficient production of hydrogen from seawater is within reach. In addition, their system offers the possibility of producing pure oxygen. After all, it is released at the anode.
Fears
Jolanda van Medevoort from Wageningen University has reservations about this finding. This might be an interesting solution, you answer, but the researchers aren’t entirely clear on the effectiveness and sustainability of their system. They themselves indicate that the membrane captures almost all chlorine ions. “In the long run, you end up with ions near your electrodes that you don’t want there.” It also expects the membranes to provide additional resistance, which in turn will lead to higher energy consumption and thus lower efficiency.
She completed her own electrolysis project last year in which yield loss was handled differently. She says electrolysis always releases heat. You can use this heat to produce pure, distilled water. In our demonstration project, Sea2H2, we have shown that net energy consumption is then negligible.” A follow-up project will show whether such efficiencies can also be achieved in an industrial-scale facility.
Read also:
A new wind farm above Wadden will produce hydrogen gas
A new wind farm above Wadden will produce not electricity, but hydrogen gas. So Schiermonnikoog’s controversial cable drilling seems off the table. The Wadden Society remains concerned.

“Total coffee specialist. Hardcore reader. Incurable music scholar. Web guru. Freelance troublemaker. Problem solver. Travel trailblazer.”






More Stories
GALA lacks a chapter on e-health
Weird beer can taste really good.
Planets contain much more water than previously thought