Philips Temporarily Stops Selling New Sleep Apnea Devices in the US The company agreed to this in a settlement with the US regulatory agency FDA.

“Passionate analyst. Thinker. Devoted twitter evangelist. Wannabe music specialist.”
 Actor Alain Delon’s dog was not given an injection to be buried together: what are our rules?
Actor Alain Delon’s dog was not given an injection to be buried together: what are our rules?
 Bitcoin price rises after new jobs data from US
Bitcoin price rises after new jobs data from US
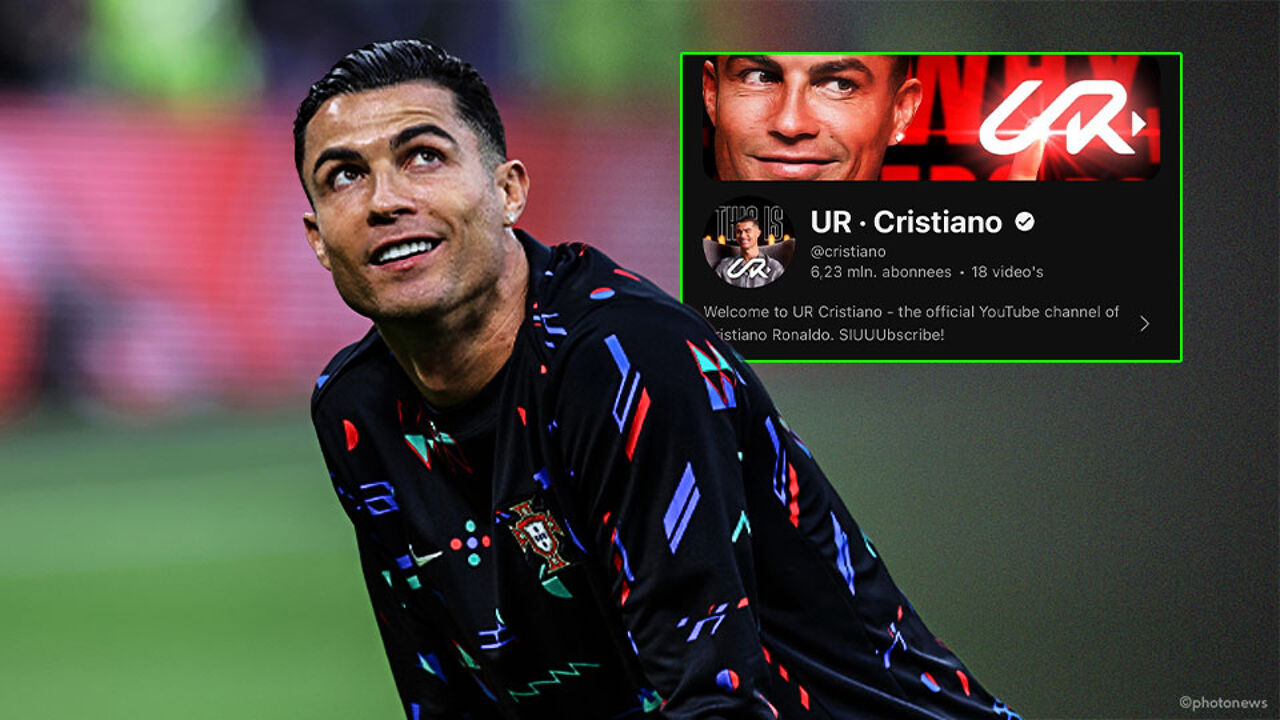 Subscribe! Cristiano Ronaldo is now also breaking a world record with… a brand new YouTube channel
Subscribe! Cristiano Ronaldo is now also breaking a world record with… a brand new YouTube channel
 GALA lacks a chapter on e-health
GALA lacks a chapter on e-health
 Comet Tsuchinshan-Atlas is ready to shine this fall
Comet Tsuchinshan-Atlas is ready to shine this fall
 Actor Alain Delon’s dog was not given an injection to be buried together: what are our rules?
Actor Alain Delon’s dog was not given an injection to be buried together: what are our rules?
 Bitcoin price rises after new jobs data from US
Bitcoin price rises after new jobs data from US
 Subscribe! Cristiano Ronaldo is now also breaking a world record with… a brand new YouTube channel
Subscribe! Cristiano Ronaldo is now also breaking a world record with… a brand new YouTube channel
 GALA lacks a chapter on e-health
GALA lacks a chapter on e-health
 Comet Tsuchinshan-Atlas is ready to shine this fall
Comet Tsuchinshan-Atlas is ready to shine this fall

29 jan 2024 om 07:47 Update: 10 minuten geleden
Philips Temporarily Stops Selling New Sleep Apnea Devices in the US The company agreed to this in a settlement with the US regulatory agency FDA.
More Stories
Cooperation between the US and China ensures more stable corporate finance – FM.nl
New US peace proposal for Gaza war ‘may be too smart for either side to say no’
Bitcoin weathers bankruptcy storm in US